
Overview
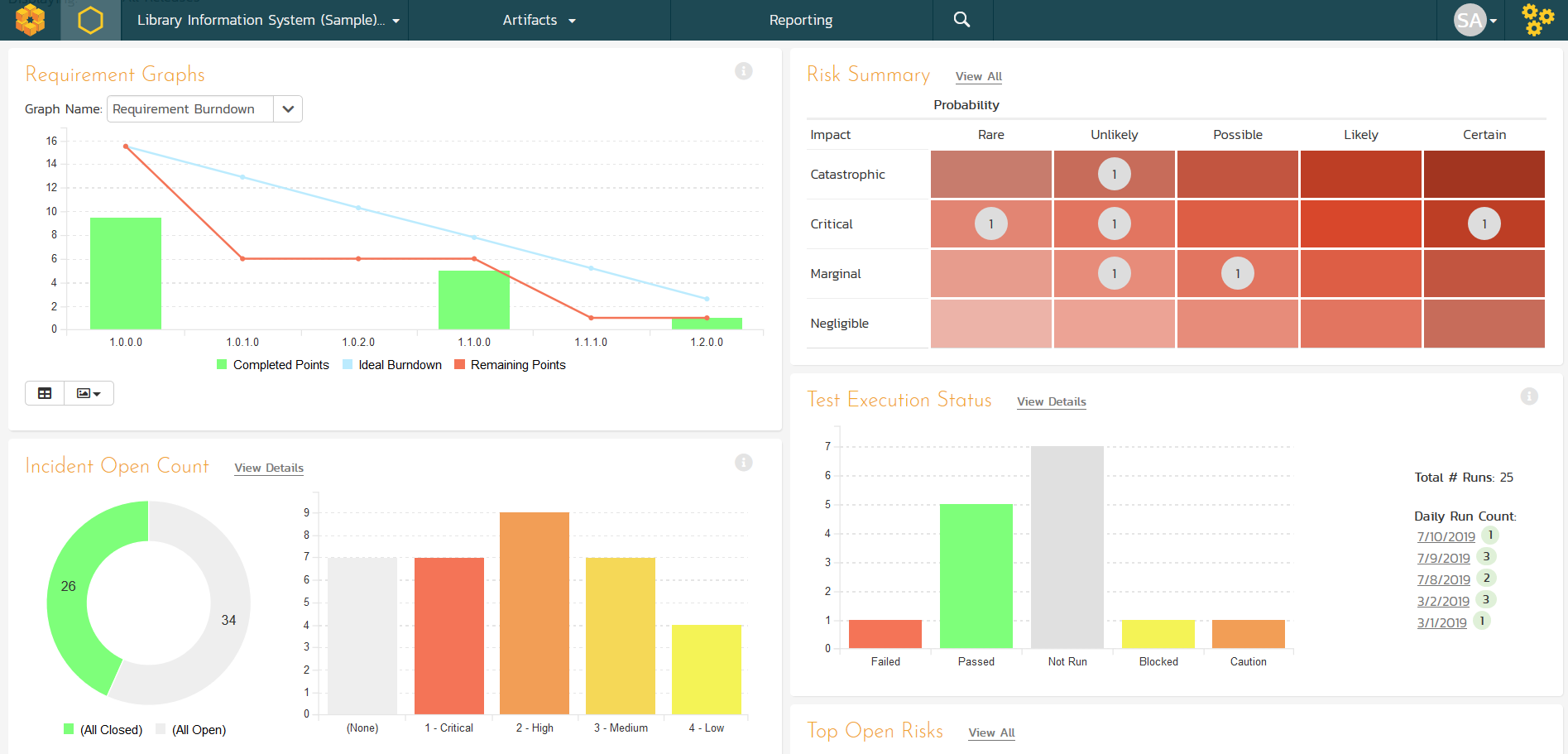
SpiraPlan Centralized Quality Dashboards
SpiraPlan dashboards give life sciences teams real-time traceability, risk and test status, and audit-ready visibility for faster, compliant releases.

SpiraPlan Centralized Quality Dashboards
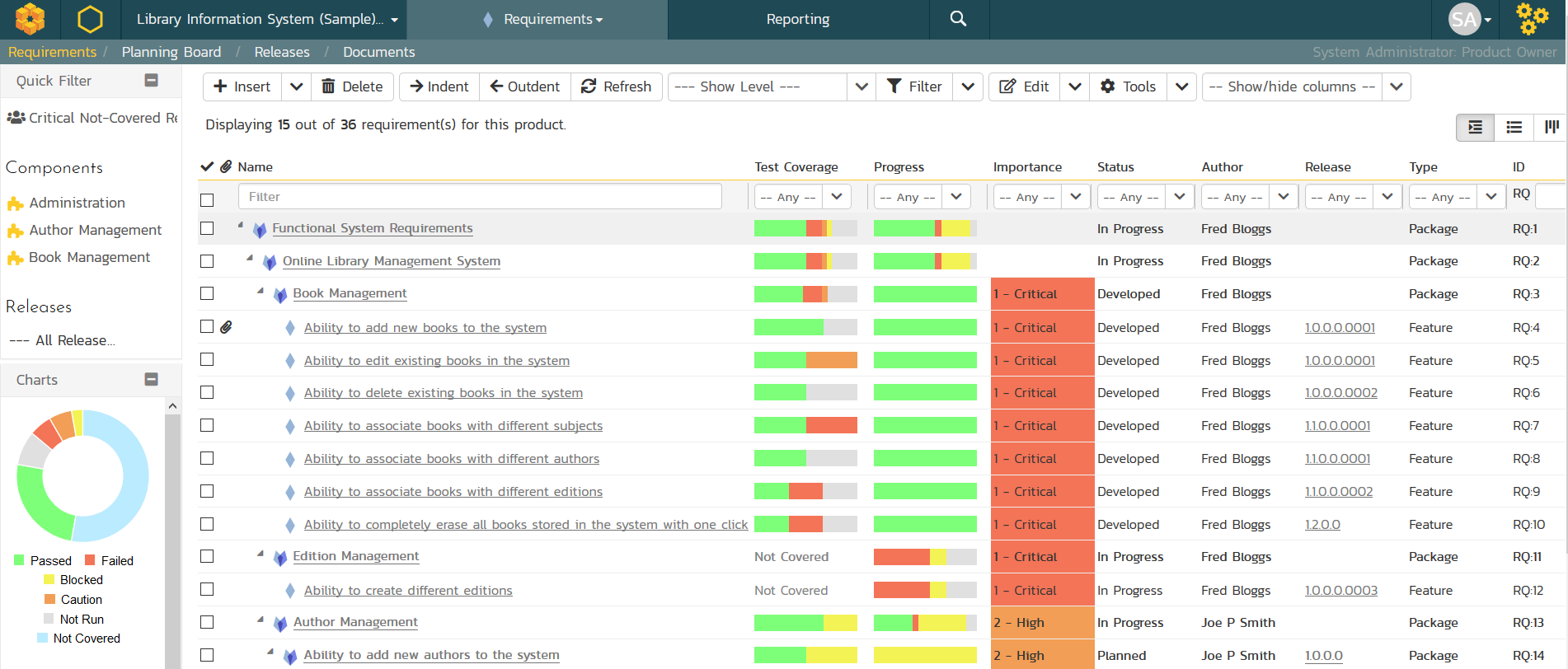
SpiraPlan Requirements Traceability
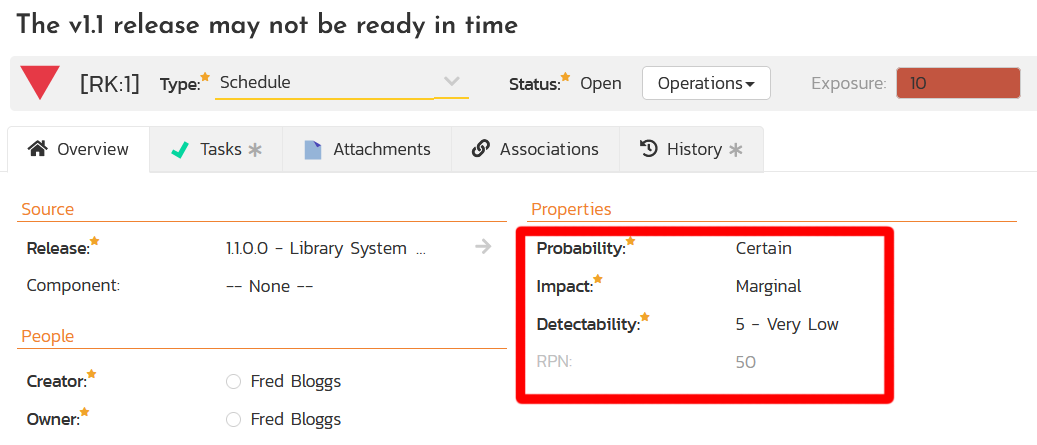
SpiraPlan Risk Management with FMEA Support
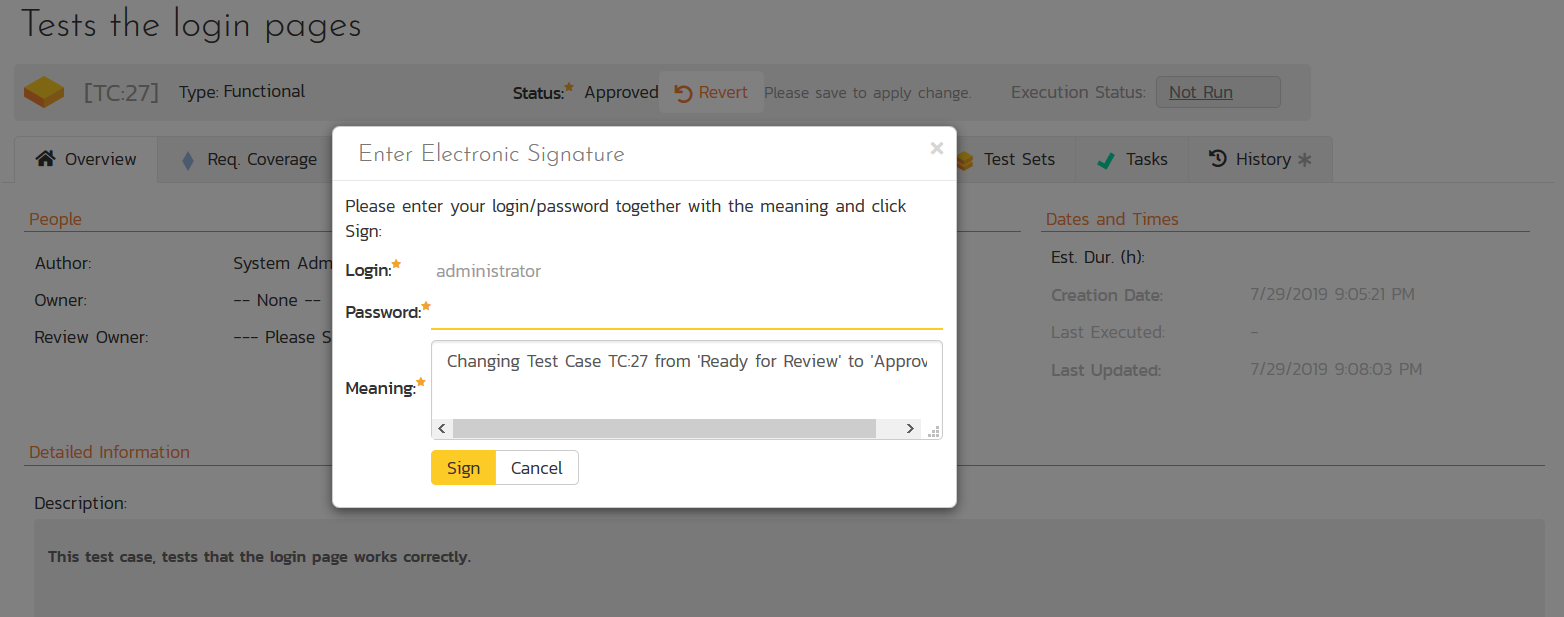
SpiraPlan Quality Management with E-Signatures
Rapise Automated Testing & Validation of Systems
The Future of Regulated Software Is Connected.
Healthcare and life sciences teams are building more digital systems than ever from medical device software and patient applications to lab systems and cloud-based platforms. As delivery accelerates, expectations for FDA, GxP, 21 CFR Part 11, HITRUST, and HIPAA compliance continue to grow.
Traditional validation workflows rely on manual steps and disconnected tools, creating gaps that slow releases and increase audit risk. To keep pace, organizations need connected lifecycle systems that provide visibility across development, testing, and validation.
Connected software lifecycle management integrates requirements, testing, validation, risks, and documentation into a single workflow. It makes compliance part of everyday work, supports continuous release cycles, and ensures teams always know the state of validation.
Key Capabilities Built for Healthcare and Life Sciences • Streamlined validation workflows with integrated testing. • Built-in 21 CFR Part 11–compliant signatures for regulated approvals. • Secure, HIPAA-compliant cloud or on-premise storage for all validation artifacts. • Unified visibility from requirements through test scenarios, execution, and results. • Automatic, secure logs capturing all user actions, changes, and timestamps. • AI Tools that generate test scenarios and scripts to reduce manual effort.
This connected approach gives teams full lifecycle visibility while reducing manual documentation and audit preparation.
Use cases
Clinical Information Systems
The Inflectra suite helps Clinical Information Systems teams deliver and maintain compliant, reliable software by managing requirements, risks, test cases, defects, and release readiness in one governed lifecycle with full traceability and audit trails. With Rapise-driven automation and integrated reporting, teams can validate changes faster, produce defensible test evidence, and reduce downtime and patient-safety risk by catching regressions early and prioritizing the highest-impact fixes.
Regulatory Submission
The Inflectra suite helps life sciences teams support clinical trial submissions by maintaining end-to-end traceability and controlled change management across trial-system requirements, validation tests, defects, and approvals—creating a defensible audit trail for submission readiness. By automating validation with Rapise and consolidating evidence and reporting in Spira, teams reduce documentation overhead, improve data integrity and consistency, and accelerate readiness for regulatory review.
Security and Regulatory
The Inflectra suite helps healthcare organizations meet security and regulatory obligations by enforcing governed SDLC/ALM processes—role-based access, approvals, traceability, and audit trails—across requirements, risks, tests, defects, and releases. By centralizing evidence and automating validation where appropriate (e.g., with Rapise), teams can demonstrate control effectiveness faster, reduce compliance effort, and improve the security posture of systems that handle sensitive patient data.
Details
Introducing multi-product solutions
You can now purchase comprehensive solutions tailored to use cases and industries.

Products included


Features and programs
Financing for AWS Marketplace purchases

Pricing
Custom pricing options
Integration guide
SpiraPlan helps life sciences teams manage regulated product delivery by enforcing requirements traceability, risk-based planning, controlled change management, and complete audit trails across the full lifecycle. Rapise supports validation by automating functional and regression testing with repeatable evidence (test scripts, execution logs, and results) that can be tied directly back to requirements and defects for end-to-end compliance reporting. Together, they reduce validation effort and documentation overhead while improving release quality and inspection readiness through centralized visibility and controlled, defensible test evidence.
How can we make this page better?
